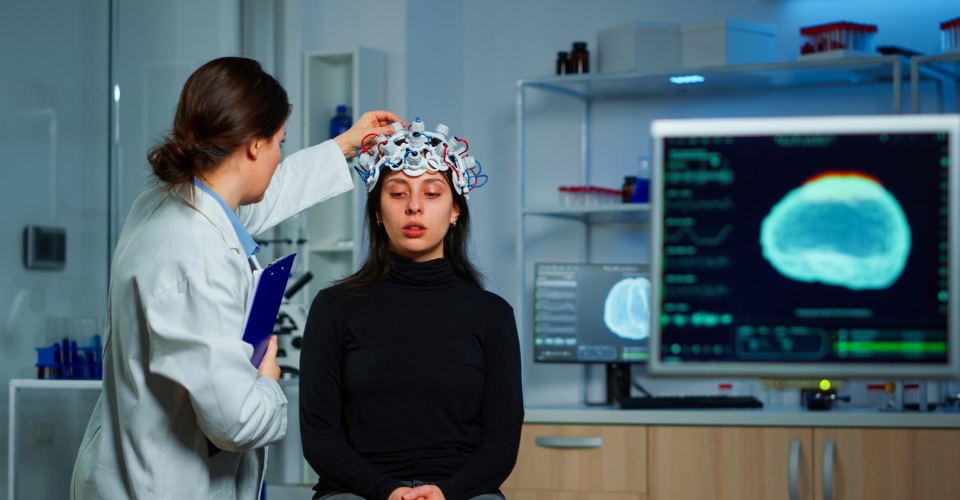
In a groundbreaking development, Cybin Inc. has unveiled the long-awaited interim findings from its Phase 2 clinical trial for CYB003, a proprietary deuterated psilocybin analog.
These results are nothing short of remarkable, indicating a swift, substantial, and statistically significant reduction in depression symptoms, observed just three weeks after a single 12mg dose when compared to a placebo.
The Phase 2 clinical trial was meticulously designed to assess the efficacy of CYB003, with a primary focus on the reduction of depression symptoms specifically at the three-week mark following a single administration.
To achieve this, the study employed the Montgomery–Åsberg Depression Rating Scale (MADRS), which includes ten clinician-administered items to measure various depressive symptoms.
MADRS is widely recognized and accepted by regulatory authorities worldwide as a standardized metric for clinical testing. It comprises items that encompass mood-related sadness, sleep and appetite changes, concentration difficulties, and more. The scoring system utilizes a scale from 0 to 6 for each item, leading to a total score range of 0 to 60.
For the CYB003 study, the mean baseline total scores on the MADRS were 32.6 for the active group and 33.3 for the placebo group.
CYB003 Demonstrates Remarkable Outcomes
The primary efficacy endpoint of the study was set at three weeks to evaluate changes in Major Depressive Disorder (MDD) symptoms, measured by alterations in the MADRS total score from the baseline. The results exceeded expectations, with participants who received CYB003 showcasing a remarkable superiority.
A substantial 14.08-point difference was observed between the control and treatment group (p=0.0005, Cohen’s d=2.15). It’s crucial to note that a p-value below 0.05 indicates statistical significance, and values below 0.001 are considered highly statistically significant.
These findings have stirred excitement in the field of mental health treatment. The results suggest that CYB003, a proprietary deuterated psilocybin analog developed by Cybin, has the potential to significantly alleviate depression symptoms.
This promising outcome could be a game-changer in the treatment of Major Depressive Disorder, offering a more rapid and effective solution compared to traditional treatments.
The study also sheds light on the importance of investigating alternative treatments for mental health conditions. Major Depressive Disorder affects millions of individuals worldwide, and traditional therapies don’t always provide the desired results.
Cybin’s innovative approach might offer new hope for those struggling with depression.
The significant difference in MADRS total scores between the active and placebo groups indicates that CYB003 can produce substantial and rapid improvements in depressive symptoms. This outcome may have far-reaching implications for individuals dealing with depression and potentially lead to a paradigm shift in the treatment of this mental health condition.
While the clinical trial results are undoubtedly exciting, more research and testing are necessary to validate the efficacy and safety of CYB003 fully. Cybin will continue its efforts to gather comprehensive data to support the potential approval and deployment of this groundbreaking treatment.
If CYB003 continues to demonstrate its effectiveness in further studies, it could become a key player in the evolving landscape of mental health treatments.
The findings from this Phase 2 trial offer a glimpse of a brighter future for individuals facing Major Depressive Disorder. As the research progresses and the potential of CYB003 becomes clearer, there is renewed hope that a new, more effective treatment option may soon be available to those who need it most.
In conclusion, the interim results from Cybin’s Phase 2 clinical trial for CYB003 are highly promising. The substantial reduction in depression symptoms observed in just three weeks after a single 12mg dose highlights the potential of this deuterated psilocybin analog to revolutionize the treatment of Major Depressive Disorder.
While further research is required, these findings offer hope for a more efficient and rapid solution to a condition that affects countless individuals. The success of CYB003 could mark a significant turning point in the field of mental health treatment.
With mental health conditions on the rise worldwide, innovative and effective treatments are urgently needed. Cybin’s commitment to advancing the understanding of CYB003’s potential is a step in the right direction, offering hope to those who may benefit from this groundbreaking treatment.
As the research continues, the possibility of a more effective approach to addressing Major Depressive Disorder comes into focus.